
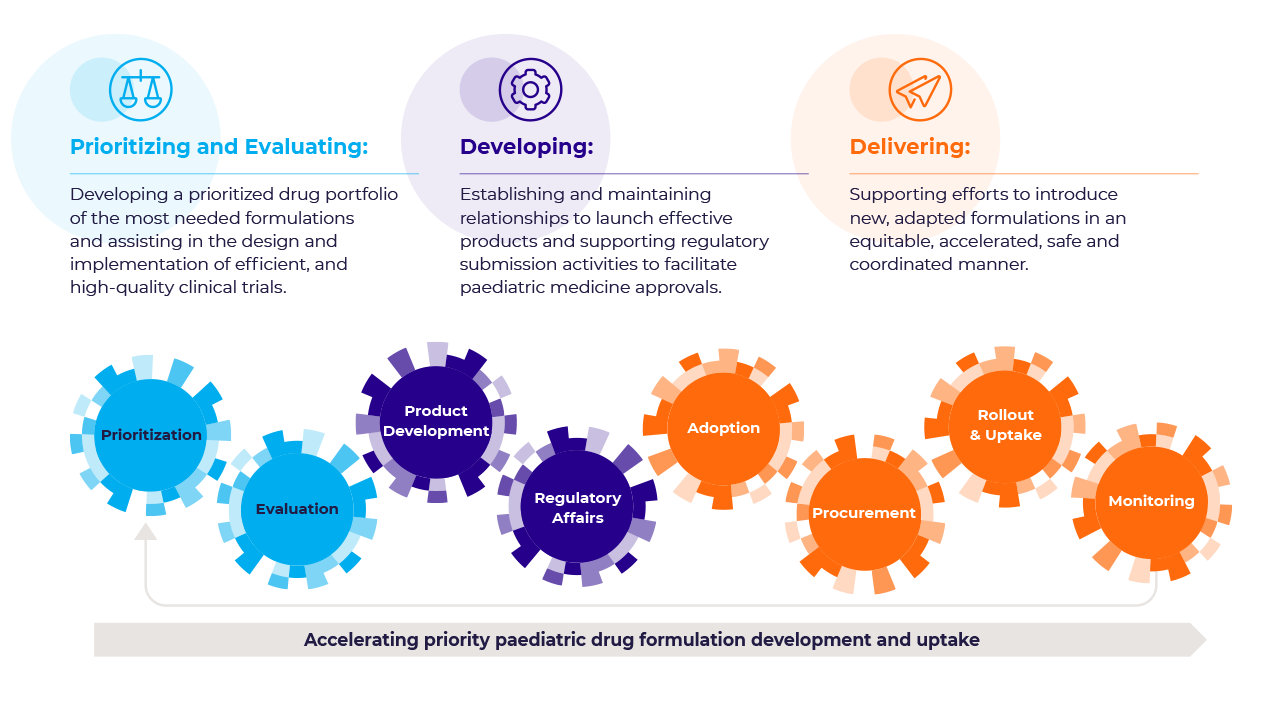
How we work
GAP-f is a collaborative framework created to provide access to better paediatric medicines more rapidly. This is achieved by prioritizing products, streamlining the generation of clinical evidence, incentivizing manufacturers, accelerating product development and introduction and coordinating procurement and roll-out as needed. GAP-f formalizes collaborations across sectors to ensure accelerated development and uptake of the most needed drugs and formulations for children. It does so by:
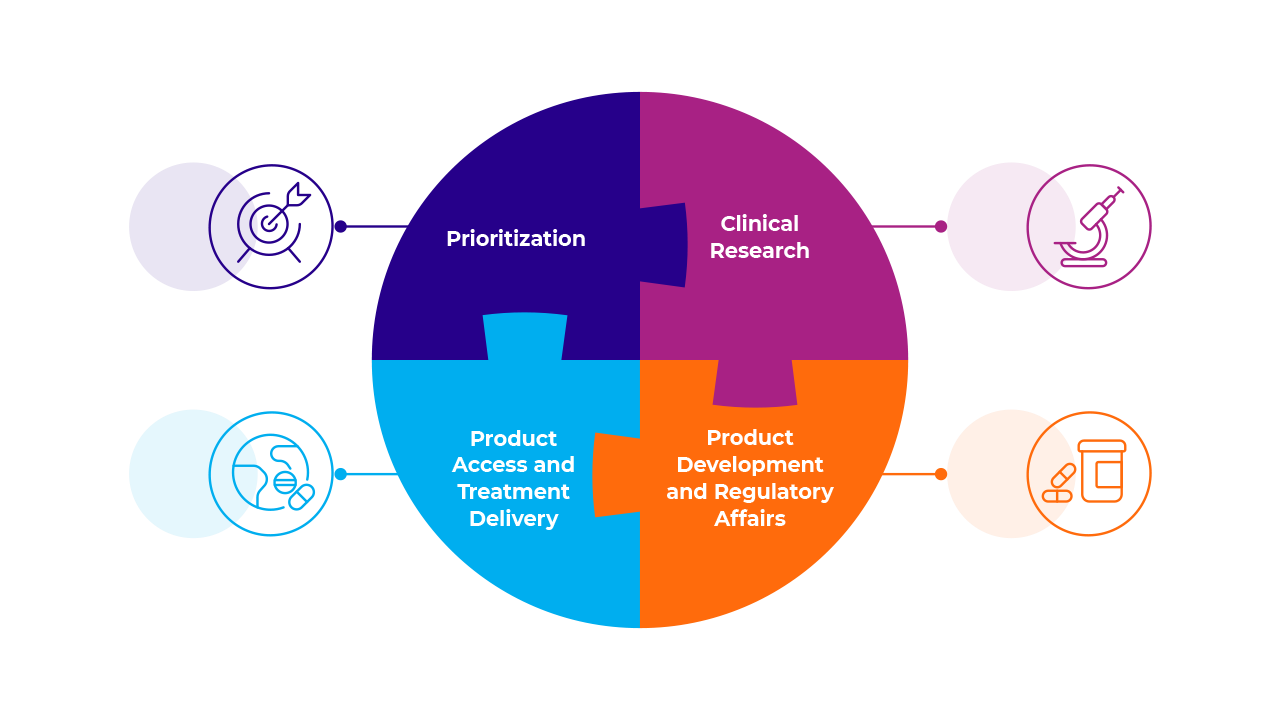
The Network functions through four Working Groups, which work across the product life cycle. These are the Prioritization Working Group, Clinical Research Working Group, Product Development and Regulatory Affairs Working Group and Product Access and Treatment Delivery Working Group. Each Network Member contributes to at least one or more Working Group, depending on their organizational expertise.
Stakeholder engagement is a critical function to achieve GAP-f’s overarching mission and individual work stream priorities. Through information sharing, coordination and collaboration with stakeholders, GAP-f will be better positioned to address key challenges and opportunities for the development and scale up of better medicines for children. GAP-f connects and interacts with external stakeholders with the specific purpose of working collaboratively to share information, build trust, and identify and address solutions to accelerate access to optimal formulations and medicines for children. Four strategic forums, the Funders and Investors Forum for Paediatric Medicines; an Industry Forum; a Civil Society and Community Engagement Platform and close collaboration with the WHO Paediatric Medicines Regulators’ Network are mechanisms through which GAP-f leverages the expertise and resources needed to target and optimize the investment of time, personnel, effort and funding.
GAP-f engages with an Advisory Committee convened ad hoc to guide GAP-f on strategic directions through its evolution. The Advisory Committee comprises of independent leaders from the global health community experienced with all phases of the product life cycle and complements the expertise of the Strategic and Coordination Committee.
