
The second national TB inventory study in Indonesia
Background and context
Indonesia’s first national tuberculosis (TB) inventory study was implemented in 2017, under the leadership of the national TB programme (NTP) and the National Institute of Health Research and Development (NIHRD). The objective was to measure the level of underreporting of people diagnosed with TB to the national TB surveillance system. The best estimate of the overall level of underreporting was 41% (95% confidence interval [CI]: 36–46%). Using capture–recapture modelling, the TB incidence rate was estimated to be 319 (95% CI: 290–349) per 100 000 population in 2017 (1).
Based on these results, the NTP implemented policies and interventions to reduce underreporting:
- a new TB surveillance system (SITB) was developed and introduced;
- there were intensified efforts to promote and reinforce the 2016 Health Ministerial Decree on mandatory notification of TB cases by all health care providers and for all forms of TB (previously, only bacteriologically confirmed cases of pulmonary TB were emphasized);
- cooperation with the Health Social Security Agency (BPJS Kesehatan) for the reporting of new TB cases was strengthened;
- active case finding interventions were expanded; and
- engagement with the private health sector was improved.
During the coronavirus disease (COVID-19) pandemic, Indonesia was one of the countries that experienced the largest reductions (both absolute and relative) in notifications of people newly diagnosed with TB, in 2020 and 2021. The number of TB case notifications officially reported to the World Health Organization (WHO) fell from 559 847 in 2019 to 384 025 (a reduction of 31%) in 2020, with a partial recovery to 432 577 in 2021 (still 23% below the level in 2019). Subsequently, there was a strong post-COVID-19 rebound, to 708 658 case notifications in 2022 and 804 836 in 2023.
To assess the level of underreporting of people diagnosed with TB post-pandemic and to estimate TB incidence, a repeat national TB inventory study was implemented in 2023. This was done by the Directorate General of Disease Prevention and Control of the Ministry of Health, in collaboration with the Agency for Health Policies Development (BKPK).
Methods and main results
The study methods – from design through to implementation, analysis and reporting of results – followed WHO guidance (2).
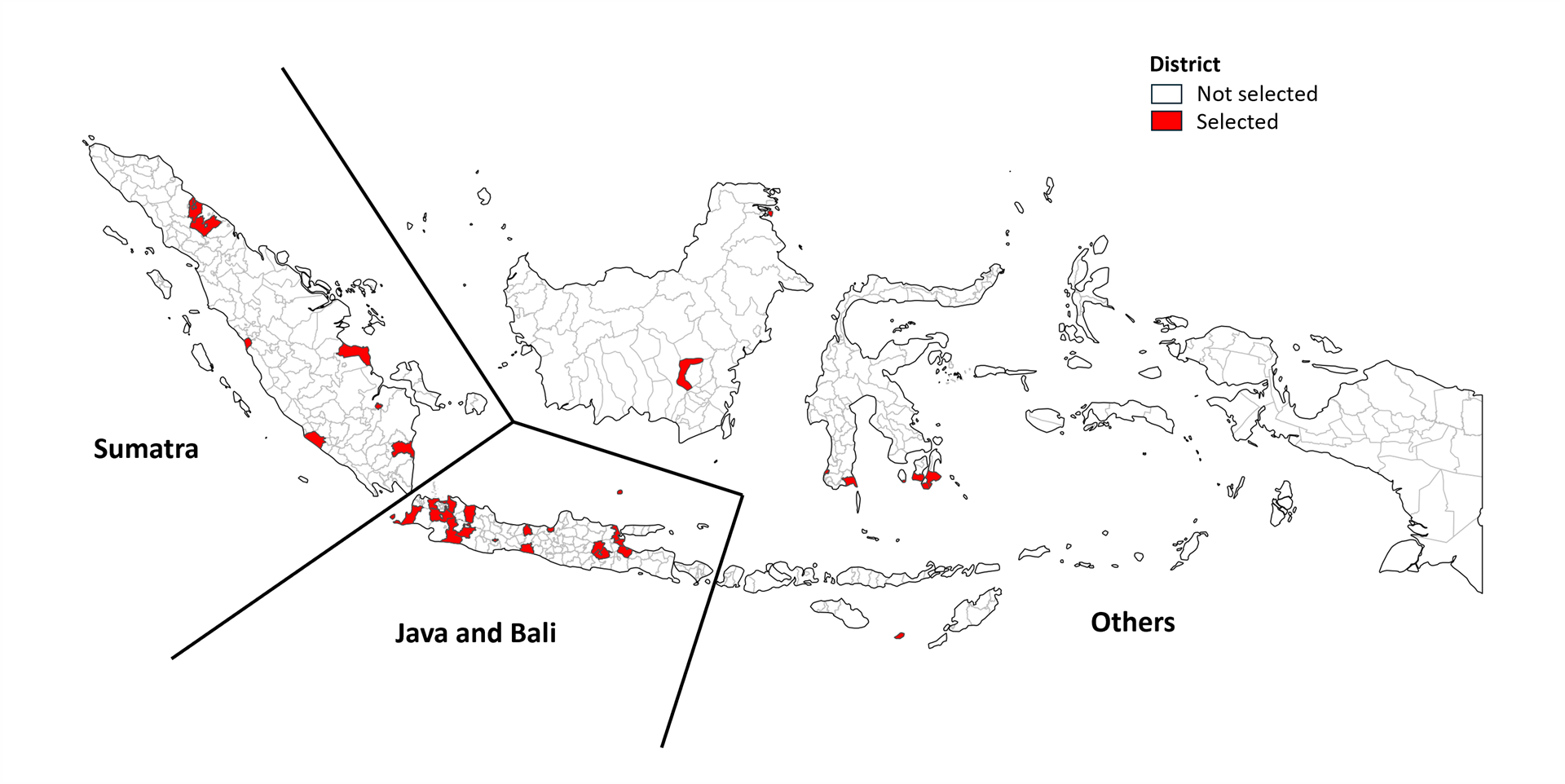
The study design was similar to the first inventory study in 2017. A random, nationally representative sample of 31 districts (out of a total of 514) was selected from across the country, using a stratified cluster-sampling design (Fig. 1). There were three strata: Sumatra, Java/Bali and other regions/Eastern Indonesia (KTI). The 31 districts accounted for 18.5% of the national population. All health care providers of TB services in the 31 districts were mapped, with a total of 6362 health care services enumerated. Among those providers, 2502 (39%) were eligible for inclusion in the study (i.e. reported having diagnosed or treated at least one person with TB in the previous 3 months), and among those, 2153 (86%) participated in the study.
During the study period (1 September 2023 to 30 November 2023) and after deduplication of records for the same person, a total of 61 634 individuals were identified as having been diagnosed with TB in the 31 districts, based on four separate data sources: SITB, laboratories included in the study, non-laboratory public providers included in the study and non-laboratory private providers included in the study. Record linkage using probabilistic matching was used to identify which of the TB cases identified during the inventory study had been reported to the SITB database.
Of the 61 634 people diagnosed with TB during the study period, 51 448 had been recorded in the SITB database (Fig. 2).
| Data sources | Number of TB cases |
|---|---|
| SITB (unique) | 51 448 |
| Study (unique) | 50 595 |
| Non-laboratory publica | 31 397 |
| Non-laboratory privateb | 19 467 |
| Laboratory (public and private) | 1130 |
| SITB – study (unique) | 61 634 |

a Primary health care facilities, hospitals and clinics.
b Hospitals, clinics and general practitioners.
Overall, the estimated underreporting of detected TB cases was 15.6% (95% CI: 13.4–18.2%). There was important variation among different types of health facilities, with the rate varying from 8% in puskesmas (health centres), to 28% in clinics and up to 55% in laboratories. Underreporting was significantly higher in private facilities, for people with clinically diagnosed TB (as opposed to bacteriologically confirmed TB) or extrapulmonary TB, and in children.
In 2023, the level of underreporting was 2.6 times lower than in 2017, a testament to the success of the interventions and policies implemented after the first national TB inventory study.
Capture–recapture modelling was used to estimate the level of undiagnosed TB, and in turn to derive estimates of TB incidence from TB notification data (using estimates for the level of both underreporting and underdiagnosis). It was estimated that 14% (95% CI: 7–20%) of incident TB cases were not diagnosed in 2023. As a result, the incidence rate in 2023 was estimated at 394 (95% uncertainty interval [UI]: 363–428) per 100 000 population.
Fig. 3 compares the WHO estimates of TB incidence published in this report (which include use of a country-specific dynamic model for 2020–2023) with the TB incidence rate estimated using results from the TB inventory study. Interestingly, the two independent estimates for 2023 are similar, although those derived from the inventory study are slightly higher. The results from the inventory study provided a robust external validation of the model-based estimates using empirical data generated at country level. The next step will be to directly incorporate the inventory study result in the country-specific model, to refine and update WHO estimates of TB incidence for Indonesia.

Conclusions and next steps
Indonesia is the first country to repeat a national TB inventory study. The study generated high-quality data, demonstrated the feasibility of successfully implementing a repeat national TB inventory study, and allowed a direct and independent estimate of TB incidence in 2023 that was consistent with the current model-based estimate published by WHO.
Following official and wide dissemination of findings in June 2024, results are being used to strengthen TB diagnosis, treatment and reporting at national and district levels. Further joint work between WHO and the NTP of Indonesia will be undertaken in the coming year, so that inventory study results can be directly used to produce WHO estimates of TB incidence. A study report is currently being finalized and results will be summarized in a paper for a peer-reviewed journal.
References
Global tuberculosis report 2018. Geneva: World Health Organization; 2018 (https://iris.who.int/handle/10665/274453).
Assessing tuberculosis under-reporting through inventory studies. Geneva: World Health Organization; 2012 (https://iris.who.int/handle/10665/78073).
