Pharmacokinetics and dosing
Pregnant women have historically been excluded from clinical trials to prevent eventual drug-related adverse effects on the women and her pregnancy. However, the lack of data in pregnancy is problematic as it may prevent the use of newer more effective treatment due to the lack of data on safety, pharmacokinetics and the related optimal dosing in this population.
Pharmacokinetics is defined as “what the body does with a drug”.
Pharmacokinetics studies the absorption, distribution, metabolism/biotransformation and excretion (ADME) of medicines.
The aim is to generate effective but non-toxic exposure to a medicine in the body. This means that the plasma concentrations of a drug should not be too low, which may lead to reduced efficacy, or too high, which may lead to toxicity.
Figure 1: Changes in absorption, distribution, metabolism/biotransformation and excretion during pregnancy
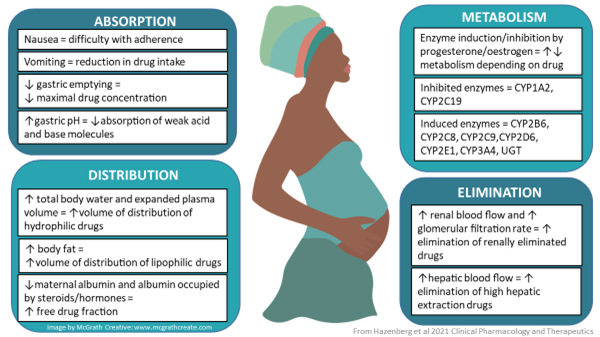
These alterations may affect the efficacy of a medicine if concentrations achieved are too low, or may cause toxicity if concentrations are too high.
Therefore, it is important to study the effect of pregnancy on the pharmacokinetics of medicines. For some medicines, like anti-epileptic drugs and some antiretrovirals, it is known that the exposure to the drug in pregnancy is lower and sometimes too low to provide good efficacy. This may lead to the decision to increase the dose (in the case of anti-epileptics) or to avoid use of the drug in pregnancy (in the case of cobicistat-boosted antiretrovirals).
Lactation
When a person takes medicines during the lactation (breastfeeding) period, the child may also be exposed to these medicines through breastmilk.
It is important to know whether and how much of a medicine passes into breastmilk and what the exposure of the child is.
By assessing the milk/plasma ratio of a medicine, we can predict how much a child will ingest. From this information the relative child dose (the dose the child gets relative to the dose of the woman) can be calculated. In general, a medicine is deemed to be safe in breastfeeding if this relative child dose is <10% of the maternal dose.
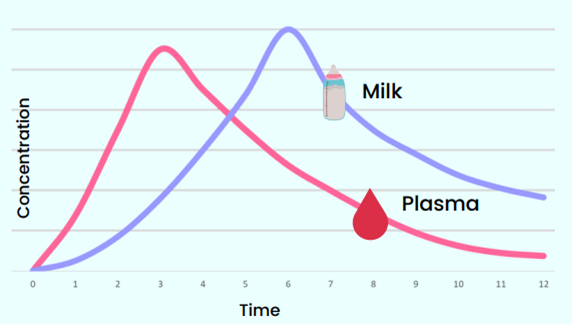
Figure 2 shows that the milk/plasma ratio depends on the time interval at which the sample is collected after the medicine intake by the person who is lactating. Therefore, it is critical to document the time at which the blood and milk samples have been collected relative to the time of the last drug intake by the woman.
Figure 2: Milk/plasma ratio (Catriona Waitt, MPRINT)
The information in this toolkit aims to inform researchers on the general basic principles regarding the conduct of pharmacokinetic studies in pregnancy and lactation. We hope that this toolkit will promote the inclusion of pharmacokinetic sampling in studies conducted during pregnancy and lactation, for example in a sub-study. Every medicine needs a tailor-made approach, and inclusion of a clinical or non-clinical pharmacology expert in the study team is highly recommended.
Case studies
The following section describes two case studies illustrating differences in the boosting effect during pregnancy and the impact of the immature activity of drug-metabolizing enzymes in the neonate on the elimination of strand transfer inhibitors (INSTIs).
Pharmacokinetic boosting effect during pregnancy
Protease inhibitors including the INSTI elvitegravir require coadministration with a pharmacokinetic booster to achieve target drug exposure. Ritonavir and cobicistat are equally strong inhibitors of cytochrome P450 (CYP) 3A4, the main enzyme metabolizing protease inhibitors and elvitegravir, and therefore were shown to be equivalent pharmacokinetic enhancers for darunavir, atazanavir and elvitegravir in non-pregnant adults. However, physiological changes in pregnancy seem to impact ritonavir- and cobicistat-boosted regimens differently. Boosting with cobicistat has been shown to result in a 77% reduction in elvitegravir trough concentrations (Ctrough) with 85% of pregnant women having concentrations below the effective elvitegravir concentration (1). Furthermore, boosting with cobicistat has been shown to result in a more pronounced decrease in darunavir or atazanavir Ctrough compared to ritonavir boosting (i.e., boosting with cobicistat resulted in a 71-92% decrease in Ctrough vs 24-64% with ritonavir boosting) (2-4). These findings led the regulatory agencies to advise against cobicistat-boosted regimens in pregnancy due to the risk of suboptimal drug exposure and the related risk of virologic failure and potential development of drug resistance.
The difference in the boosting effect during pregnancy has several potential explanations. First, pregnancy related physiological changes cause a more pronounced decrease in cobicistat exposure compared to ritonavir so that cobicistat concentrations are more likely to drop below the half maximal inhibitory concentration for CYP3A4 inhibition during the dosing interval, resulting in a decreased boosting effect [4]. Second, ritonavir is a more robust booster, as CYP3A4 inhibition is unchanged even at reduced ritonavir exposures. Atazanavir Ctrough was shown to be similar when boosted with 50 mg vs 100 mg ritonavir (i.e., 0.16 vs 0.13 mg/L) whereas a reduction in cobicistat dose from 150 to 100 mg led to a significantly lower atazanavir Ctrough (i.e., 0.13 vs 0.08 mg/L). Boosting with ritonavir has been shown to better counteract the effect of inducers (4). Altogether, these data suggest that ritonavir boosting is more robust and therefore should be favored during pregnancy.
The revised recommendation against the use of cobicistat containing regimens during pregnancy was made several years after the cobicistat-containing regimens were first approved for use in adults thereby highlighting the long-standing issue of the time gap between the approval of drugs and the availability of pharmacokinetic studies for the safe use of drugs during pregnancy. Pregnant persons are generally excluded from drug development trials, furthermore pharmacokinetic data during pregnancy are not required for the approval of new drugs. Thus, all effort should be made to enroll pregnant persons in the early phase of drug trials (phases IIb and III) to characterize drug exposure and safety to ensure that these data are available at the time of initial approval. Furthermore, modelling studies should be used more commonly to further inform drug exposure and dosing in this special population.
Immaturity of drug-metabolizing enzymes’ activity and prolonged elimination of INSTIs in the neonate
INSTIs are metabolized to various extent by uridine diphosphate-glucuronosyltransferase (UGT)1A1: raltegravir > cabotegravir, dolutegravir > bictegravir. This enzyme is also responsible for the metabolism of bilirubin which has been shown to be slow immediately after birth due to a low activity of UGT1A1 at birth, but which increases nearly 100-fold during the first 14 weeks of life (5). Thus, immaturity of UGT1A1 activity can result in prolonged elimination of INSTIs in neonates. Examples of changes in the expression of other metabolic enzymes with age are shown in Figure 4.
Raltegravir elimination has indeed been shown to be highly variable and extremely prolonged in some infants (median elimination half-life (range): 26.6 hours (9.3-184 hours)) in a study evaluating the washout pharmacokinetic of raltegravir in infants born to pregnant women on a raltegravir-based regimen (6). An initial dosing study of two single raltegravir doses during the first week of life, one during the first 48 hours of life and a second around 7 days of life, confirmed the slow raltegravir elimination immediately after birth, but which accelerated during the first week of life. These data combined with pharmacokinetic data in infants older than one month led to an eight-fold daily dosing increase from 1.5 mg/kg once daily during the first week of life, to 3 mg/kg twice daily for weeks 2-4 and then 6 mg/kg twice daily at 4 weeks of life to maintain therapeutic raltegravir concentrations in full-term infants (7). A modeling study has suggested that prematurity further reduces raltegravir clearance so that a modified raltegravir dosing regimen will be needed to avoid elevated plasma raltegravir concentrations (8).
Dolutegravir elimination has been shown to be prolonged in newborns with a median elimination half-life of 33 hours (compared to a median maternal postpartum half-life of 13.5 hours) as a result of UGT1A1 activity immaturity (9). The transfer of dolutegravir into the placenta and breastmilk coupled with the delayed infant clearance can lead to significant infant dolutegravir exposures (3-8% of maternal plasma concentrations) in the early postpartum period (10). However, a modelling study suggests that breastfeeding has little contribution to infant dolutegravir plasma exposure after cessation of maternal dolutegravir, which wanes in the postpartum period as transplacental dolutegravir is cleared (11).
Bictegravir elimination is also prolonged in infants after birth with a median (Q1-Q3) elimination half-life of 33.2 (25.7-45.9) hours (12). In a small breastfeeding study, bictegravir concentrations in infants (measured one month after birth) were shown to be approximately 2% of those in the maternal plasma but higher than those in the breastmilk (13). This observation likely relates to the immaturity of drug metabolizing enzymes activity which can lead to some drug accumulation through breastfeeding although the estimated infant daily dose from breastfeeding remains low.
Finally, limited data are available for cabotegravir. A case report suggests that cabotegravir concentrations in the newborn one day after birth could be comparable to those measured in the plasma of the mother (14). Studies are warranted to evaluate the impact of UGT1A1 activity immaturity on cabotegravir washout pharmacokinetics in neonates.
References
- Bukkems V, Necsoi C, Tenorio CH, Garcia C, Rockstroh J, Schwarze-Zander C, et al. Clinically significant lower elvitegravir exposure during the third trimester of pregnant patients living with human immunodeficiency virus: data from the pharmacokinetics of antiretroviral agents in HIV-infected pregnant women (PANNA) Network. Clin Infect Dis. 2020; 71(10): e714-717.
- Momper JD, Wang J, Stek A, Shapiro DE, Scott GB, Paul ME, et al. Pharmacokinetics of darunavir and cobicistat in pregnant and postpartum women with HIV. AIDS. 2021; 35(8):1191-1199.
- Momper JD, Wang J, Stek A, Shapiro DE, Powis KM, Paul ME, et al. Pharmacokinetics of atazanavir boosted with cobicistat in pregnant and postpartum women with HIV. J Acquir Immune Defic Syndr. 2022; 89(3): 303-309.
- Bukkems VE, Colbers A, Marzolini C, Molto J, Burger DM. Drug-drug interactions with antiretroviral drugs in pregnant women living with HIV: are they different from non-pregnant individuals? Clin Pharmacokinet. 2020; 59(10): 1217-1236.
- Kawade N, Onishi S. The prenatal and postnatal development of UDP-glucuronyltransferase activity towards bilirubin and the effect of premature birth on this activity in the human liver. Biochem J. 1981; 196(1): 257-60.
- Clarke DF, Acosta EP, Rizk ML, Bryson YJ, Spector SA, Mofenson LM, et al. Raltegravir pharmacokinetics in neonates following maternal dosing. J Acquir Immune Defic Syndr. 2014; 67(3):310-5.
- Clarke DF, Mirochnick M, Acosta EP, Capparelli E, Chain A, Teppler H et al. Use of modeling and simulation to determine raltegravir dosing in neonates: a model for safely and efficiently determining appropriate neonatal dosing regimens: IMPAACT P1110. J Acquir Immune Defic Syndr. 2019; 82(4): 392-8.
- Clarke DF, Lommerse J, Acosta EP, Cababasay MP, Wang J, Spector SA, et al. Impact of low birth weight and prematurity on neonatal raltegravir pharmacokinetics: Impaact P1077. J Acquir Immune Defic Syndr. 2020; 85(5):626-634.
- Mulligan N, Best BM, Wang J, Capparelli EV, Stek A, Barr E, et al. Dolutegravir pharmacokinetics in pregnant and postpartum women living with HIV. AIDS. 2018; 32(6): 729-737.
- Waitt C, Orrell C, Walimbwa S, Singh Y, Kintu K, Simmons B, et al. Safety and pharmacokinetics of dolutegravir in pregnant mothers with HIV infection and their neonates: a randomized trial (DolPHIN-1 study). PLoS Med. 2029; 16(9):e1002895.
- Dickinson L, Walimbwa S, Singh Y, Kaboggoza J, Kintu H, Sihlangu M, et al. Infant exposure to dolutegravir through placental and breast milk transfer: a population pharmacokinetic analysis of DolPHIN-1. Clin Infect Dis. 2020; 73(5): e1200-e1207.
- Powis KM, Pinilla M, McMorrow F, Stek A, Brooks KM, Shapiro DE, et al. Pharmacokinetics and safety of bictegravir in pregnant and postpartum persons with HIV and their infants. J Acquir Immune Defic Syndr. 2025; 98(3): 300-307.
- Aebi-Popp K, Kahlert CR, Crisinel PA, Decosterd L Saldanha SA, Hoesli I, et al. Transfer of antiretroviral drugs into breastmilk: a prospective study from the Swiss Mother and Child HIV Cohort Study. J Antimicrob Chemother. 2022; 77(12): 3436-3442.
- van der Wekken-Pas L, Weiss F, Simon-Zuber C, Sebisch R, Wiese C, van Leeuwen E, et al. Long-acting injectable cabotegravir and rilpivirine in a pregnant woman with HIV. Clin Infect Dis. 2024; 79(6): 1468-71.
Resources
Module 3 of this toolkit focuses on research in pregnant and breastfeeding women. Key considerations are given about the timing of pharmacokinetic studies in pregnancy and lactation and which data are key to collect.
Report and papers generated after the “Approaches to Optimize and Accelerate Pharmacokinetic Studies in Pregnant and Lactating Women” workshop in 2019, organised by WHO and IMPAACT
This workshop brought together the different stakeholders with the aim to generate consensus on how to optimize and accelerate pharmacokinetic studies in pregnant and lactating women. This workshop was focussed around antiretrovirals for treatment of HIV, but the main outcomes can be applied more generally to treatment of infectious diseases or chronic diseases that need to be treated during pregnancy.
Presentations at the United States Food and Drug Administration (FDA) virtual workshop “Pharmacokinetic Evaluation in Pregnancy” 2022
The objectives of this online workshop organized by the FDA were to:
- Review the general landscape of existing regulatory, scientific, and ethical considerations for drug development in pregnant individuals
- Describe study design considerations for trials collecting PK data in pregnant individuals
- Discuss key aspects of utilizing modelling and simulation to inform study design and dosing considerations for pregnant individuals
- Explore innovative approaches to data generation and analysis in pregnant individuals
The paper summarising the outcomes of the workshop has been published: Pharmacokinetic Evaluation in Pregnancy— Current Status and Future Considerations: Workshop Summary
The presentations can be found here.
US FDA guidance documents
The US FDA also issued guidance documents for the conduct pharmacokinetic studies in pregnancy and lactation.
Studies and programmes
IMPAACT 2026
IMPAACT 2026 is a Phase IV, prospective, pharmacokinetic (PK) study of selected antiretroviral (ARV) and anti-tuberculosis (TB) drugs during pregnancy and postpartum. The study is designed to evaluate the following: the PK of ARV medicines used in clinical care during pregnancy and postpartum; the PK of ARVs when used in combination with first-line TB medicines in clinical care during pregnancy and postpartum; the PK of second-line TB medicines when used in clinical care during pregnancy and postpartum; the kinetics of placental and breastmilk transfer of long-acting injectable ARVs after maternal dosing during pregnancy; and the kinetics of breastmilk transfer of other select ARVs from mother to child during breastfeeding.
- Study website
- Protocol
- Manual of procedures
- Informed consent form (see protocol, pages 144-221)
- Laboratory Processing Charts (available from the study website, under Documents)
P1026S
P1026s was a Phase IV, prospective pharmacokinetic (PK) study of selected ARV drugs currently used in the clinical care of HIV-infected pregnant women during pregnancy and postpartum. This study was designed to evaluate the following: the pharmacokinetics of antiretroviral medicines when used alone or co-administered with tuberculosis medicines during pregnancy; the pharmacokinetic parameters of lopinavir/ritonavir and atazanavir/ritonavir/tenofovir in women postpartum before and after starting hormonal contraceptives; and the concentrations of ethinyl estradiol, etonogestrel and other progestins in women using hormonal contraceptives and protease inhibitors.
This study is now closed, but the protocol and publications can be found on the study website.
PANNA study
PANNA is the name of the study of Pharmacokinetics of newly developed ANtiretroviral agents in HIV-infected pregNAnt women (PANNA). The purpose of the study is to collect pharmacokinetic data (PK curves) in pregnant HIV-infected women using newly developed antiretroviral agents, in a European network. Information on this study and publications can be found here.
MPRINT - Improving outcomes for mothers and children through translational research
The Maternal and Pediatric Precision in Therapeutics (MPRINT) Hub serves as a national resource to aggregate, present, and expand the available knowledge, tools, and expertise in maternal and pediatric therapeutics to the broader research, regulatory science, and drug development communities. The Knowledge and Research Coordination Center at Indiana University-Ohio State University maintains a web portal to access an underlying curated knowledge base of maternal and pediatric pharmacology and therapeutics. The University of California San Diego’s and Vanderbilt University’s Center of Excellence in Therapeutics (CETs) are working to improve outcomes for maternal and pediatric populations through cutting-edge translational research. MPRINT hosts a pharmacometrics & clinical trial design scientific core. This core provides expertise in pharmacokinetic (PK) and pharmacodynamic (PD) modeling, biostatistics, and clinical trial design. They organize webinars on the subject which can be found here:
ConcePTION
ConcePTION aims to establish a trusted ecosystem that can efficiently, systematically, and in an ethically responsible manner, generate and disseminate reliable evidence-based information regarding effects of medications used during pregnancy and breastfeeding to women and their healthcare providers. This will be achieved by generating, cataloguing, linking, collecting and analysing data from pharmacovigilance, modelling, routine healthcare, and breastmilk samples through a large network. Information on this network and the output can be found here.
MILK / At The EQUATOR
ATtaining EQUity of Access TO Research (At The EQUATOR) aims at transforming research culture to ensure equity of access to research. Videos about the importance of pharmacokinetics in breastfeeding and participating in clinical trials can be found here.
Pharmacometrics Africa
Pharmacometrics Africa is a platform for interested groups to establish and run open-access quantitative clinical pharmacology educational programs in partnership with local research organisations and academic groups. The website hosts information on the pharmacokinetics of antiretrovirals in breastmilk.
Human Milk Institute
They envision a future where human milk is the first and critical foundation for human life and equitable access to better health for the global community. They are laying the foundation for achieving this vision by establishing a world-class institute and creating a sustainable model for ongoing research into human milk. The aim of the Human Milk Institute is to accelerate understanding of human milk and apply that knowledge to improve lifelong health worldwide.
Lactmed
The LactMed® database contains information on drugs and other chemicals to which breastfeeding women may be exposed. It includes information on the levels of such substances in breast milk and infant blood, and the possible adverse effects in the nursing infant. Suggested therapeutic alternatives to those drugs are provided, where appropriate. All data are derived from the scientific literature and fully referenced. A peer review panel reviews the data to assure scientific validity and currency. Drugs and Lactation Database (LactMed®) - NCBI Bookshelf (nih.gov).
Pharmacokinetics and Safety of Commonly Used Drugs in Lactating Women and Breastfed Infants (CUDDLE)
This study is conducted by the Pediatric Trials Network and aims to assess the safety of a number of commonly used off-patent medications, such as ciprofloxacin, doxycycline and amoxicillin, given to breastfeeding mothers. The study will include collection of breast milk and maternal and infant blood samples, to determine passage into breast milk and safe dosing.
Additional references
Ideal Time to Conduct a Pharmacokinetic Investigation After Delivery to Fully Capture the Effect of Pregnancy on Drug Exposure. Berton M, Stader F, Bettonte S, Battegay M, Marzolini C. Open Forum Infect Dis. 2024 Oct 15;11(10):ofae585.
Design Considerations for Pharmacokinetic Studies During Pregnancy Stika CS and Hebert MF, The Journal of Clinical Pharmacology, 2023, 63(S1) S126–S136.
Drug exposure during pregnancy: Current understanding and approaches to measure maternal-fetal drug exposure. Hudson RE. Metz TD, Ward RM, et al, Front. Pharmacol., 2023 Mar 23:14:1111601.
Disclaimer
The provision of study materials and links from the toolkit to other websites is provided for your convenience and does not indicate endorsement of those materials or sites by WHO. WHO accepts no responsibility for the validity or accuracy of their content. The mention of specific companies or of certain manufacturers' products does not imply that they are endorsed or recommended by WHO in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters.

/global-hiv-hepatitis-and-stis-programmes-(hhs)/figure-3-pharmacokinetic-boosting-during-pregnancy1fe2897d7abe4e99b4114f6b6f56319d.tmb-549v.png?sfvrsn=2a5cc779_2)
/global-hiv-hepatitis-and-stis-programmes-(hhs)/figure-4-ontogeny-of-metabolic-enzymes.tmb-768v.png?sfvrsn=6077c5c9_2)