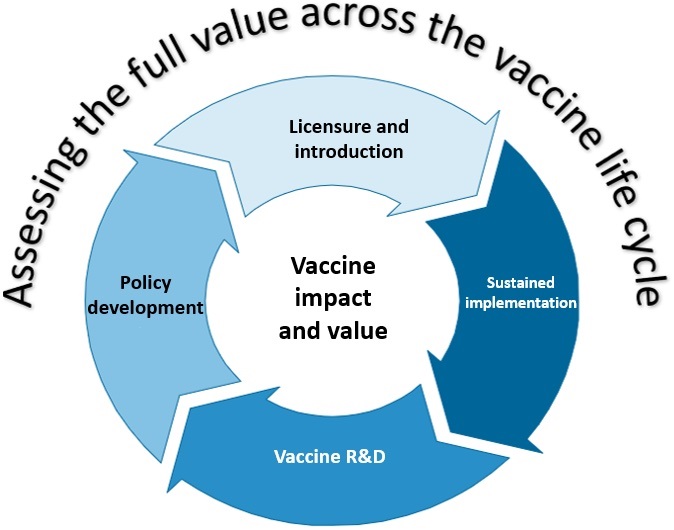
Full Value of Vaccines Assessment (FVVA)
The pathway from vaccine product concept, through development, licensure and deployment is costly and inherently risky for manufacturers, particularly for vaccines against diseases where the burden is predominantly in LMICs. To reduce the uncertainty in investment and inform potential introduction decisions, IVB has developed a framework and methodology to broaden the evaluation of ‘vaccine value’ beyond solely the demonstration of individual health benefits, to include broader socioeconomic and indirect impact(s) that the vaccine could have. This concept is known as the ‘Full Vaccine Value Assessment’ or FVVA, and is considered a critical analysis to inform prioritization for investment and eventual uptake. WHO has developed a framework to assess and communicate the FVVA, accessible here.
WHO vaccine development
In resource-constrained settings, an increasingly compelling rationale will be needed to justify the inclusion of these new vaccines within immunization programmes, over and above many other priorities. As such, the ability to guarantee the global demand, and the willingness to procure at the end of a costly product development pathway is uncertain, and vaccine manufacturers often prioritize high-income markets that offer a more immediate return on investment. The result can be a delay between vaccine licensure and access to these vaccines by low- and middle-income countries (LMICs), where there is the greatest public health need.
At WHO, the Vaccine Product & Delivery Research unit and the Value of Vaccines team jointly develop Full Value of Vaccine Assessments and support their development through 2 workstreams:
- The Vaccine Product & Delivery Research unit together with the Product Development for Vaccines Advisory Committee develops Technology Roadmaps, Preferred Product Characteristics, Vaccine Value Profiles and normative guidance to support vaccine development
- The Value of Vaccines team together with the Immunization and Vaccine related Implementation Research Advisory Committee provides advice on methods and development of Full Value of Vaccine Assessments
Contacts
Projects
